HIV : First Long-Acting Injection Approved
- Par Brenda YUFEH,
- 23 nov. 2021 10:04
- 0 Likes
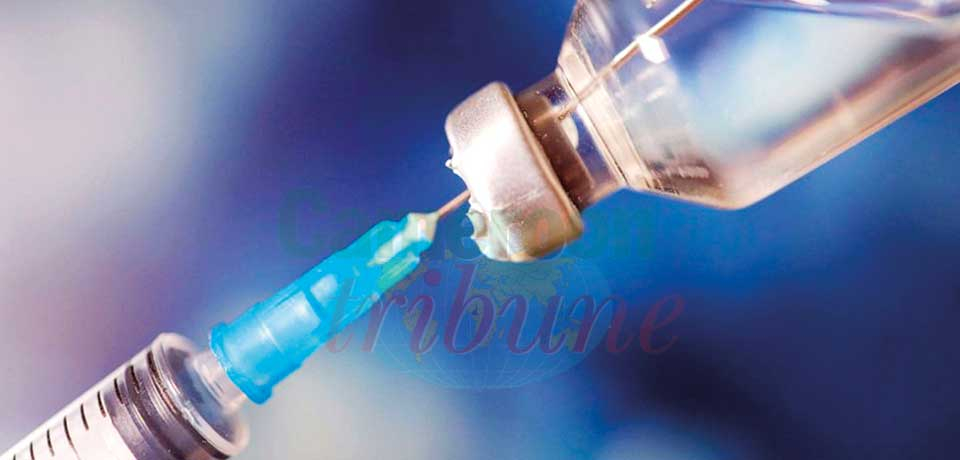
The shot called Cabenuva is a bimonthly treatment in the United Kingdom.
The United Kingdom (UK) has approved a new HIV treatment that requires an injection every other month, rather than the current routine of taking pills every day. The 1st injectable, bimonthly HIV treatment has been received with excitement from those living with HIV in the UK. The injection keeps the virus at bay, in a similar way to conventional antiretroviral drugs. Experts say it could be more convenient for many patients as it will relief them from the burden of daily oral therapy, and offer them instead only six treatments per year. The treatment, researchers say, is only suitable for those who have already achieved undetectable levels of virus in blood while taking tablets.
The UK’s National Institute for Health and Care Excellence estimated that around 13,000 people will now be eligible for cabotegravir with rilpivirine, the injectable medication. “It is an exciting and progressive step in the fight against HIV,” Dr. Todd Ellerin, Director of Infectious Diseases at South Shore Health and ABC News contributor said. The shot called Cabenuva (a combination of cabotegravir and rilpivirine) was already approved by the Food and Drug Administration (FDA) in January 2021 though with a more frequent dosage of once a month. According to Ellerin, there are hopes that by the first quarter of 2022, the FDA also approves the bimonthly injection, and with that more patients might choose this method over pills, especially if the Covid-19 situation eases.
"The medication approved in the UK will be once every two months, which makes it easier for the patients to come to the office rather than coming every month," Ellerin noted. The injection is suitable for those who prefer a more intermittent method. However, the Director of Infectious Diseases at South Shore He...
Cet article complet est réservé aux abonnés
Déjà abonné ? Identifiez-vous >
Accédez en illimité à Cameroon Tribune Digital à partir de 26250 FCFA
Je M'abonne1 minute suffit pour vous abonner à Cameroon Tribune Digital !
- Votre numéro spécial cameroon-tribune en version numérique
- Des encarts
- Des appels d'offres exclusives
- D'avant-première (accès 24h avant la publication)
- Des éditions consultables sur tous supports (smartphone, tablettes, PC)












Commentaires